Minamata’s Fight for Justice:
How Japan’s Failed Debate and Response Sparked
Local and Global Change
Industrial Revolution
The industrial revolution of the 18th-20th centuries transformed agrarian societies, increasing the demand for heavy industries, including the chemical industry, to facilitate mass production. However, factories polluted the air, water, and soil with hazardous wastes, such as mercury.
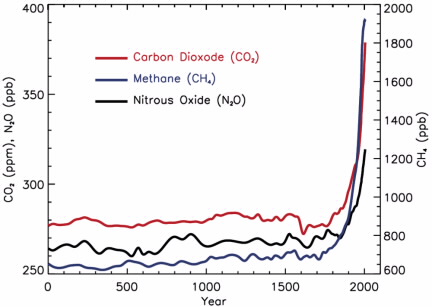
Air pollution from 0-2000, 2007, American Chemical Society
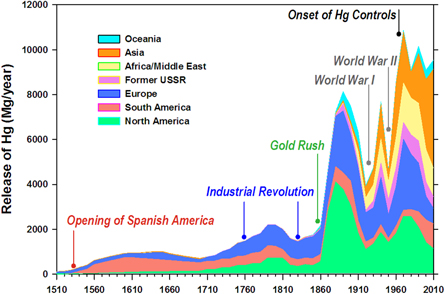
Forces influencing mercury releases from 1510-2010, 2019, Environmental Research Letters
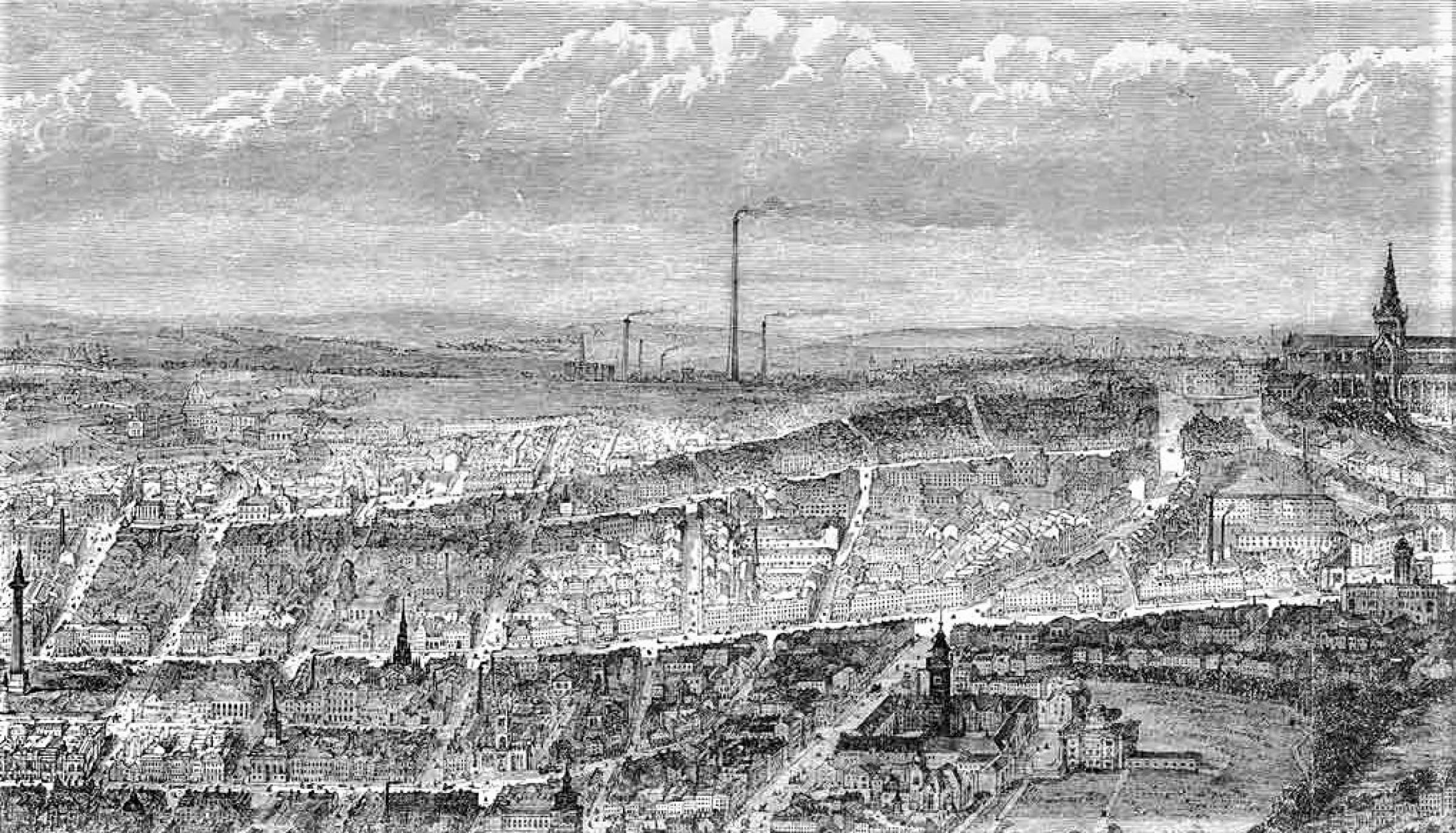
Thomas Suliman’s illustration of St. Rollox’s chemical works as published in the London Illustrated News, 1864, Lost Glasgow

Mills on the Merrimack River, circa 1908, History of Massachusetts

Potteries landscape in Bournville, 1926, Wellcome Collection
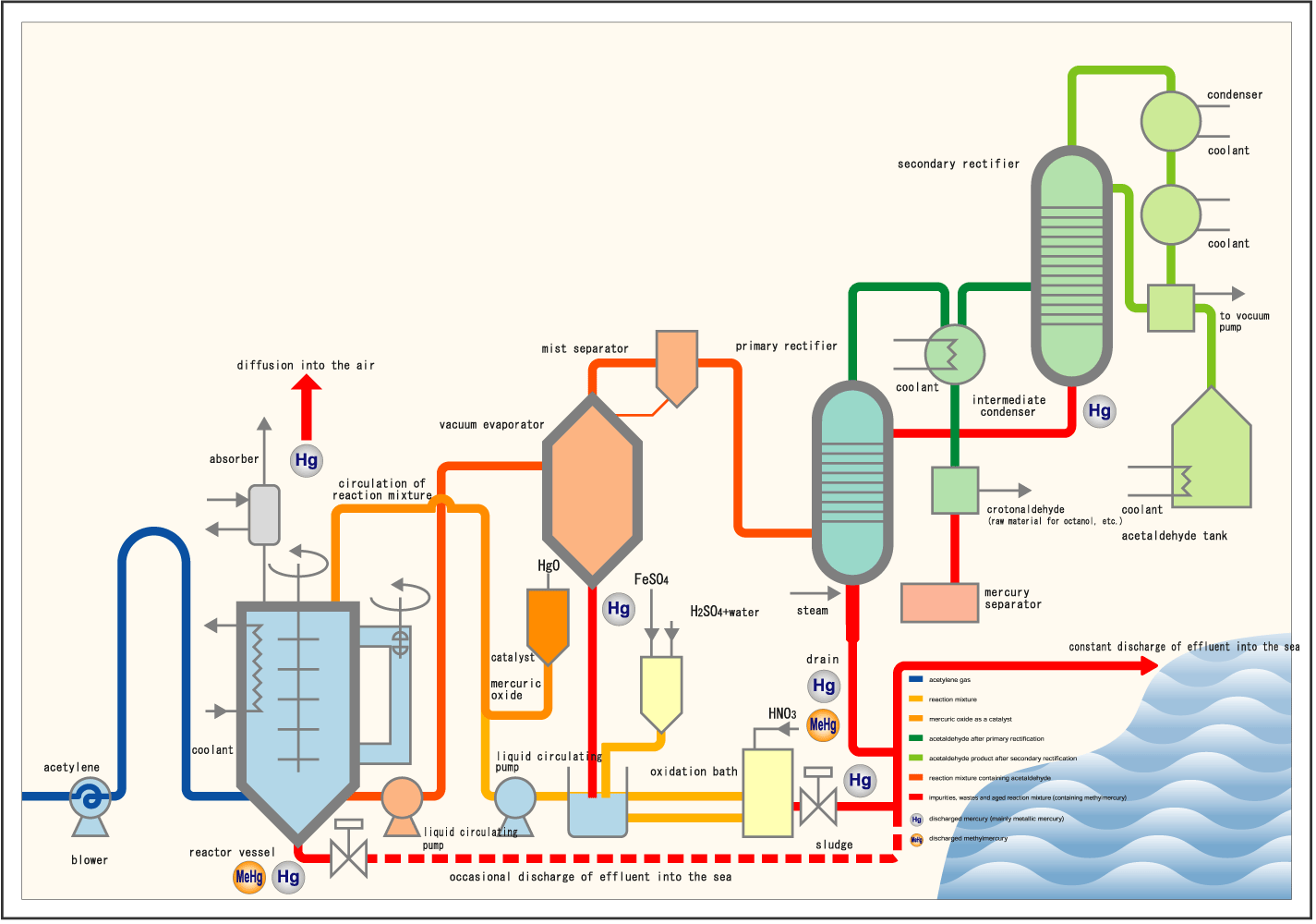
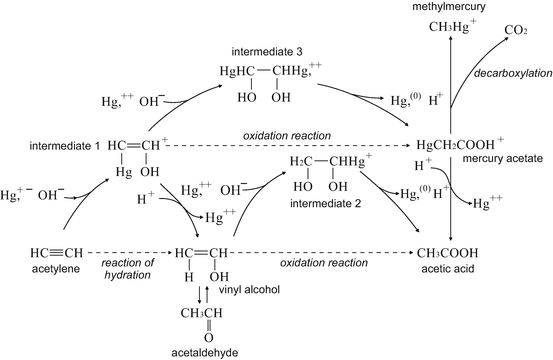
While the chemical compound acetaldehyde was first produced artificially in 1774, it was not used commercially until World War I for acetone production. However, in Minamata’s case, the industrial hydrolysis of acetylene into acetaldehyde—using mercury as a catalyst—released the byproduct of methylmercury into the environment with devastating human effects.